Abstract
Introduction
Venetoclax (VEN) is a potent B-cell lymphoma 2 (BCL-2) inhibitor and demonstrates synergistic anti-AML activity when used in combination with hypomethylating agents (HMA) such as azacitidine (AZA) and decitabine (DEC). This regimen has demonstrated high response rates and durable activity in treatment naïve (TN) older patients; however, the efficacy in relapsed and/or refractory (R/R) AML is less well characterized, with one study showing a response rate of 21%. To further characterize VEN plus HMA activity in these populations, we retrospectively reviewed the outcomes of both TN and R/R AML patients treated with VEN plus HMA at the University of California Davis Comprehensive Cancer Center (UCDCCC).
Methods
Adult patients (≥18 years) with an acute leukemia treated with VEN off protocol between January 1, 2014 through June 22, 2018 were included. Under an IRB-approved protocol, patients were retrospectively reviewed using an electronic medical record generated report. Baseline data included patient demographics, performance status, disease characteristics, prior chemotherapy, bone marrow biopsy studies and labs. Regimen data included other chemotherapy agents received, VEN dose and modifications, antifungal prophylaxis (ppx), and granulocyte colony stimulating factor (GCSF) use. Efficacy outcomes included complete remission (CR), CR with incomplete count recovery (CRi), composite CR (cCR, defined as CR + CRi), morphologic leukemia free state (MLFS), overall leukemia response (OLR, defined as cCR + MLFS), overall survival (OS), and relapse free survival (RFS). Toxicity outcomes were reviewed, including tumor lysis syndrome (TLS), febrile neutropenia (FN), and prolonged pancytopenia. All follow-up clinic visits, hospitalizations and deaths were reviewed.
Results
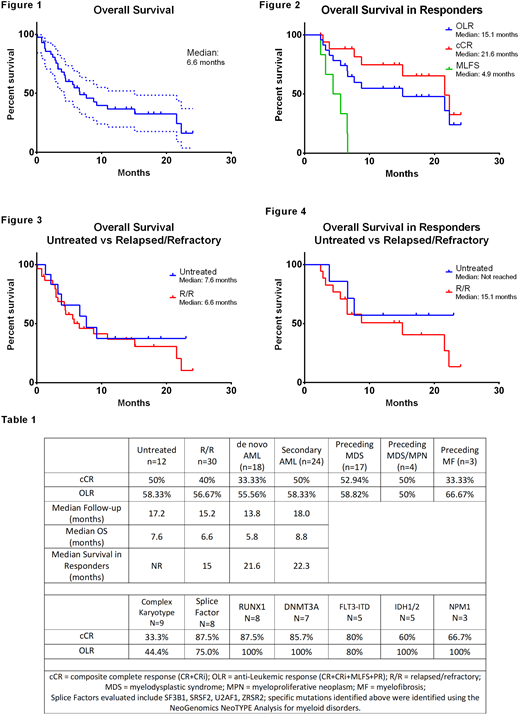
Forty-two patients were included (AML, n=41; acute undifferentiated leukemia, n=1). Median age was 67 years [25-88] and 67% were male. Eighteen (43%) had de novo AML, 17 (40%) had preceding myelodysplastic syndrome (MDS), 4 had MDS/myeloproliferative neoplasm (MPN) (10%) and 3 (7%) had primary myelofibrosis. Sixteen were TN (38%), thirteen (31%) had relapsed disease, and 13 (31%) had refractory disease. Median number of prior regimens was 1 [0-6]. Thirty-seven (88%) had an ECOG of 0 to 1 [0-3]. ELN genetic risk classification was intermediate in 19 (45%) and adverse in 22 (52%). VEN was combined with AZA in 12 (29%) and DEC in 30 (71%). Median VEN dose was 400 mg [50-800 mg]. Median follow-up was 17.2 months. For the entire study, the cCR was 43% and the OLR was 57%. See table 1 for cCR and OLR for subgroups including previously untreated, R/R, de novo, secondary AML (sAML), and various molecular and cytogenetic subgroups. Median OS was 6.2 months (Figure 1). For patients with cCR, OLR, and MLFS, median survival was 21.6, 15.1, and 4.9 months respectively (Figure 2). Median OS and median OS in responders for patients with de novo AML, sAML, untreated patients, and R/R patients is shown in Figure 3, Figure 4, and Table 1. Median number of cycles for cCR was 1 [1-6]. Of patients who obtained cCR, 6 (33%) experienced relapse of AML. Median time to relapse was 6.6 [3.4-13.9] months. Eight (19%) patients were bridged to allotransplant. Lab TLS occurred in 1 patient. Seventeen (40%) experienced prolonged pancytopenia. Eighteen (43%) had FN and 4 (22%) received GCSF. Antifungal ppx was used in 41 (98%) patients: micafungin in 17 (40%) and a non-fluconazole azole in 24 (57%). Seven (17%), of which 5 (71%) had R/R AML, were diagnosed with a fungal infection; 5 (71%) were receiving ppx azoles and 2 (29%) micafungin. Three and 6 died within 30 and 60 days of therapy initiation, respectively; all 3 patients who died within 30 days had R/R AML. The most common cause of death was refractory AML at 14 (52%) followed by infection in 9 (33%).
Conclusion
At UCDCCC, VEN in combination with an HMA is well tolerated and produces high rates of response in adult patients with AML. Response rates for TN AML, sAML and multiple molecular subgroups are consistent with prior reports, while higher than expected response rates and survival were seen in R/R AML. Responses were also seen in post-MDS/MPN and post-MF patients. In extended follow-up, survival has been durable in patients with cCR, but not MLFS. The use of VEN plus HMA combinations in adults with AML represents a viable treatment option for both TN and R/R AML.
Jonas:AbbVie: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Daiichi Sankyo: Research Funding; Genentech/Roche: Research Funding; Glycomimetics: Research Funding; Pharmacyclics: Research Funding; Tolero: Consultancy; Amgen: Consultancy; Forma: Research Funding; Incyte: Research Funding; Esanex: Research Funding; Kalobios: Research Funding; Accelerated Medical Diagnostics: Research Funding; LP Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal